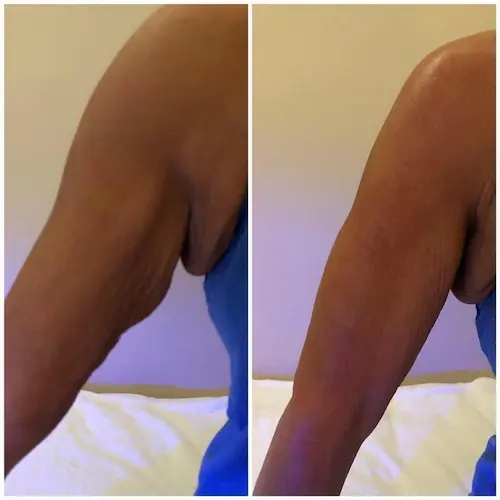
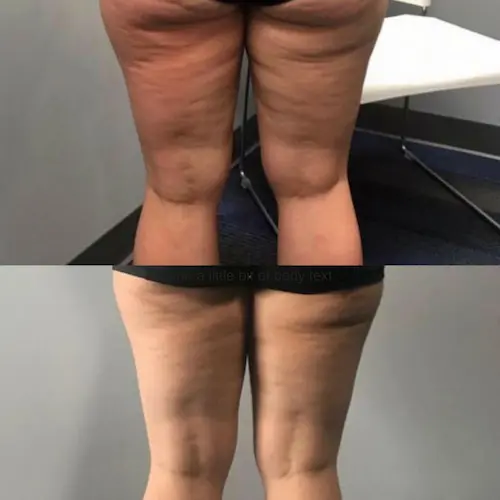
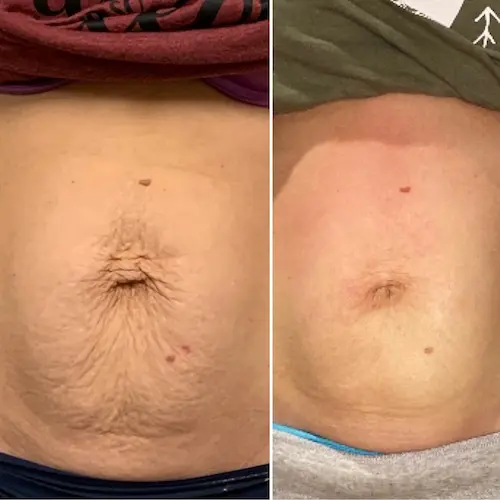
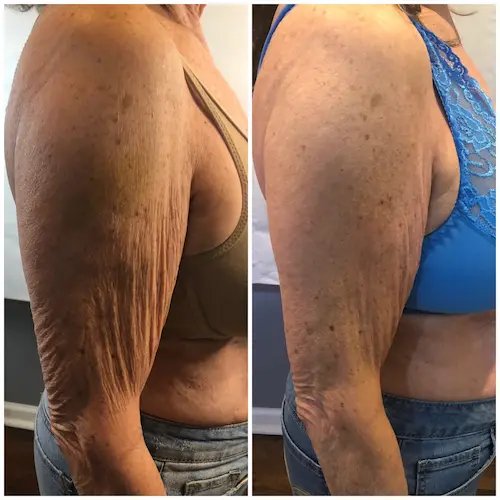
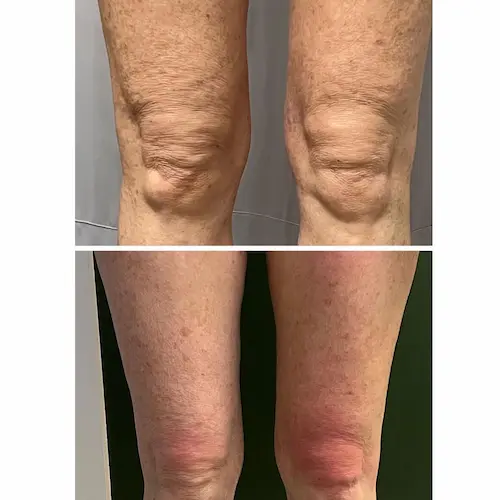
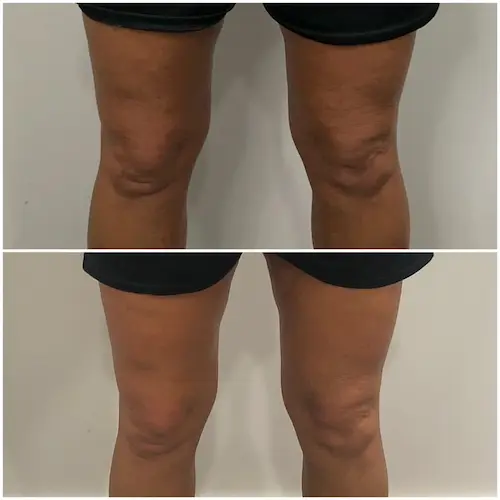
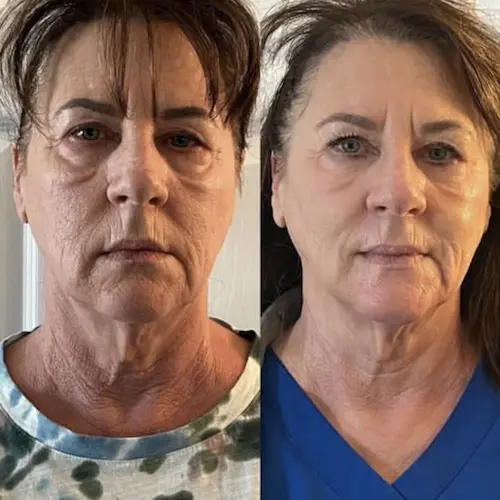
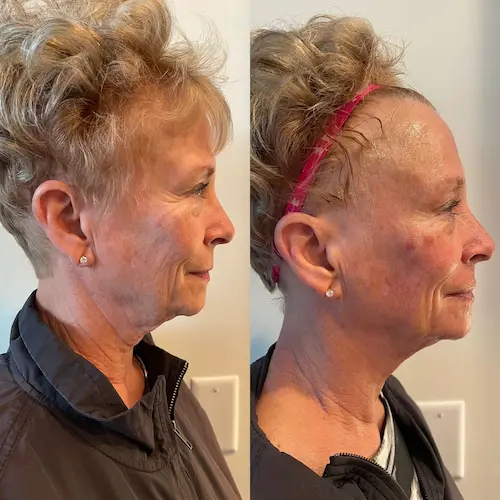
The following conditions are an absolute no for Manual/Static CryoSlimming®, Static CryoCellulite, Static CryoDrainage:
- Active Cancer
- Severe Raynaud’s Syndrome
- Severe Allergy to Cold
- Cold-related Illness (Cryoglobulinemia, Paroxysmal Cold Hemoglobinuria, Cold Agglutinin Disease)
- Lower Limb Ischemia
- Uncontrolled Diabetes or Diabetes-related complications
- Severe Kidney or Liver Disease
- Pregnancy/Breastfeeding
- Fillers in the treatment area in the past 90 days
- Bacterial and viral infections of the skin
- Active/Severe Eczema, rashes, or dermatitis in the desired treatment area
- Silicone/other implants in the desired treatment area
- Irremovable body piercings in the desired treatment area
- Incision scar(s) in the desired treatment area
- Open or infected wounds in the desired treatment area
- Impaired skin sensation in the desired treatment area
- Known sensitivity or allergy to propylene glycol
Clients with the following conditions must consult a physician before receiving Manual/Static CryoSlimming®, Static CryoCellulite, Static CryoDrainage:
- Past Cancer
- HIV/AIDS
- Progressive Diseases (including but not limited to MS, ALS, Parkinson’s, Neuropathy)
- Cardiovascular Disease
- Lymphatic Disorders
- Wound healing disorders
- Circulatory disorders
- Use of topical antibiotics in the desired treatment area
- Any surgery in the past 6 months
- Metal implants in or adjacent to the desired treatment area
- Mesh inserts in or adjacent to the desired treatment area
- Hernia in or adjacent to the desired treatment area
- Active implanted device such as pacemaker or defibrillator
- Any serious health condition not specified
The following conditions are an absolute no for Manual CryoToning®:
- Severe Raynaud’s Syndrome
- Severe Allergy to Cold
- Cold-related Illness (Cryoglobulinemia, Paroxysmal Cold Hemoglobinuria, Cold Agglutinin Disease)
- Lower Limb Ischemia
- Pregnancy/Breastfeeding
- Fillers in the treatment area in the past 90 days
- Bacterial and viral infections of the skin
- Active/Severe Eczema, rashes, or dermatitis in the desired treatment area
- Silicone/other implants in the desired treatment area
- Irremovable body piercings in the desired treatment area
- Open or infected wounds in the desired treatment area
- Impaired skin sensation in the desired treatment area
- Known sensitivity or allergy to propylene glycol
Clients with the following conditions must consult a physician before receiving a Manual CryoToning®:
- Active cancer
- Past cancer
- HIV/AIDS
- Progressive Diseases (including but not limited to MS, ALS, Parkinson’s, Neuropathy)
- Cardiovascular Disease
- Wound healing disorders
- Circulatory disorders
- Use of topical antibiotics in the desired treatment area
- Surgery in or adjacent to the desired treatment area in the past 6 months
- Metal implants in or adjacent to the desired treatment area
- Mesh inserts in or adjacent to the desired treatment area
- Hernia in or adjacent to the desired treatment area
- Active implanted device such as pacemaker or defibrillator
- Active/Severe Eczema, rashes, or dermatitis outside of the desired treatment area
- Any serious health condition not specified
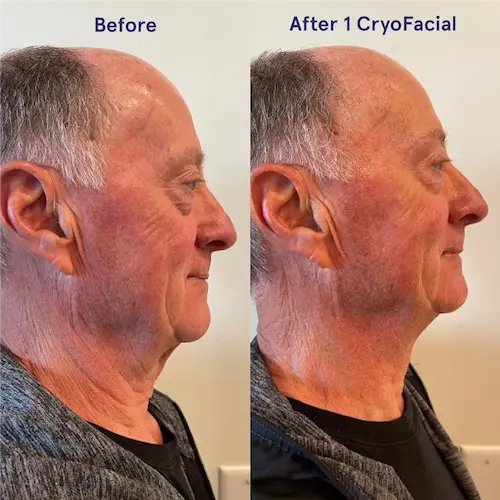
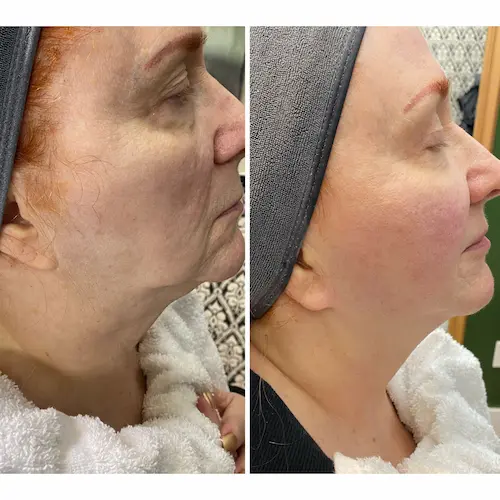
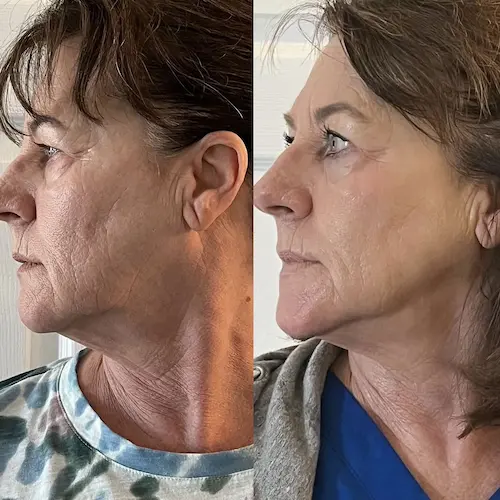
The following conditions are an absolute no for the CryoFacial:
- Severe Raynaud’s Syndrome
- Severe Allergy to Cold
- Cold-related Illness (Cryoglobulinemia, Paroxysmal Cold Hemoglobinuria, Cold Agglutinin Disease)
- Lower Limb Ischemia
- Botox in the past 30 days
- Facial fillers in the past 90 days
- PDO threads in the past 90 days
- Bacterial and viral infections of the skin
- Active/Severe Eczema, rashes, or dermatitis in desired treatment area
- Silicone/other implants in desired treatment area
- Irremovable body piercings in the desired treatment area
- Open or infected wounds in the desired treatment area
- Impaired skin sensation in the desired treatment area
- Known sensitivity or allergy to propylene glycol
Clients with the following conditions must consult a physician before receiving a CryoFacial:
- Active cancer
- Past cancer
- HIV/AIDS
- Pregnancy
- Progressive Diseases (including but not limited to MS, ALS, Parkinson’s, Neuropathy)
- Cardiovascular Disease
- Wound healing disorders
- Circulatory disorders
- Use of topical antibiotics in the desired treatment area
- Surgery in or adjacent to the desired treatment area in the past 6 months
- Metal implants in or adjacent to the desired treatment area
- Mesh inserts in or adjacent to the desired treatment area
- Hernia in or adjacent to the desired treatment area
- Active implanted device such as pacemaker or defibrillator
- Active/Severe Eczema, rashes, or dermatitis outside of the desired treatment area
- Any serious health condition not specified
The following conditions are an absolute no for Electrical Muscle Stimulation (E-Stim) [Cryoskin 4.0 ONLY]:
- Pregnancy/Breastfeeding
- Pacemaker
- Cancer
- Current or recent bleeding/hemorrhage
- Active Tuberculosis
- Thrombophlebitis or Thrombosis
- Open wounds
- Compromised Circulation
- Regenerating nerves
- Altered tissue sensation
- Impaired mental status
- Presence of any implanted electrical devices